Technology for Large-Scale Mixing Systems
For over 70 years, VMI has been developing innovative technology to optimize the manufacture of liquid and semi-solid products in the cosmetics, pharmaceutical, and specialty chemicals sectors. The expertise of our engineering offices allows us to deploy high-performance mixing installations customized to your needs.

Automation
The control systems developed by VMI are designed to meet the standards and regulations of the pharmaceuticals and cosmetics industries (GAMP5, CFR21 Part11, S88). We integrate the best Supervision, Control and Data Acquisition Systems, such as Siemens, Wonderware, and Rockwell. Our operator interfaces are complete and intuitive, with modules dedicated to your specific process needs. Our interfaces allow:
- Control over the operation and parameters of a full production line
- Programming of recipes
- Data visualization and analysis. These reports allow you to analyze the manufacturing process and identify areas for optimization: adjustment of parameters, consumption, maintenance of equipment and its components, etc.
- Batch traceability
- Reproducibility of production and cleaning processes
- Equipment durability thanks to metrology and maintenance solutions
Dosing, transfer, and storage

VMI’s mixing installations cover the entire manufacturing process. We provide turnkey fully automated solutions from dosing of ingredients to storage of the finished products. The ingredients are stored or prepared in process tanks, then dosed and transferred to the manufacturing tanks. A transfer panel controls the ingredients’ flow according to your specifications. The agitation tool is selected according to the viscosity, density, and processing required.
Our standard installations include process tanks for the finished products, featuring storage, temperature control, and suspension.

Safe integration in your production environment
Our mixing units are designed for manufacturing in controlled environments and clean rooms. Also called white rooms, these require precise materials, temperature, air quality, hygrometry, and maintenance.
Our production units can be installed in a sterile environment with a grey area for storage (raw materials, detergents, thermal control unit, CIP tanks, etc.) and maintenance access. We can also assist you in classifying hazardous locations into zones and proposing the most suitable ATEX equipment for identified explosion risks in compliance with regulations.

Homogenization technology: from simple to triple agitation
For more than 70 years, VMI has designed and manufactured a complete range of agitators to cover the most varied processes: dilution, dissolution, suspension, homogenization, dispersion, and emulsion. Our mixers and homogenizers meet both simple agitation needs and more complex processes requiring the combination of several mechanical and physical effects.
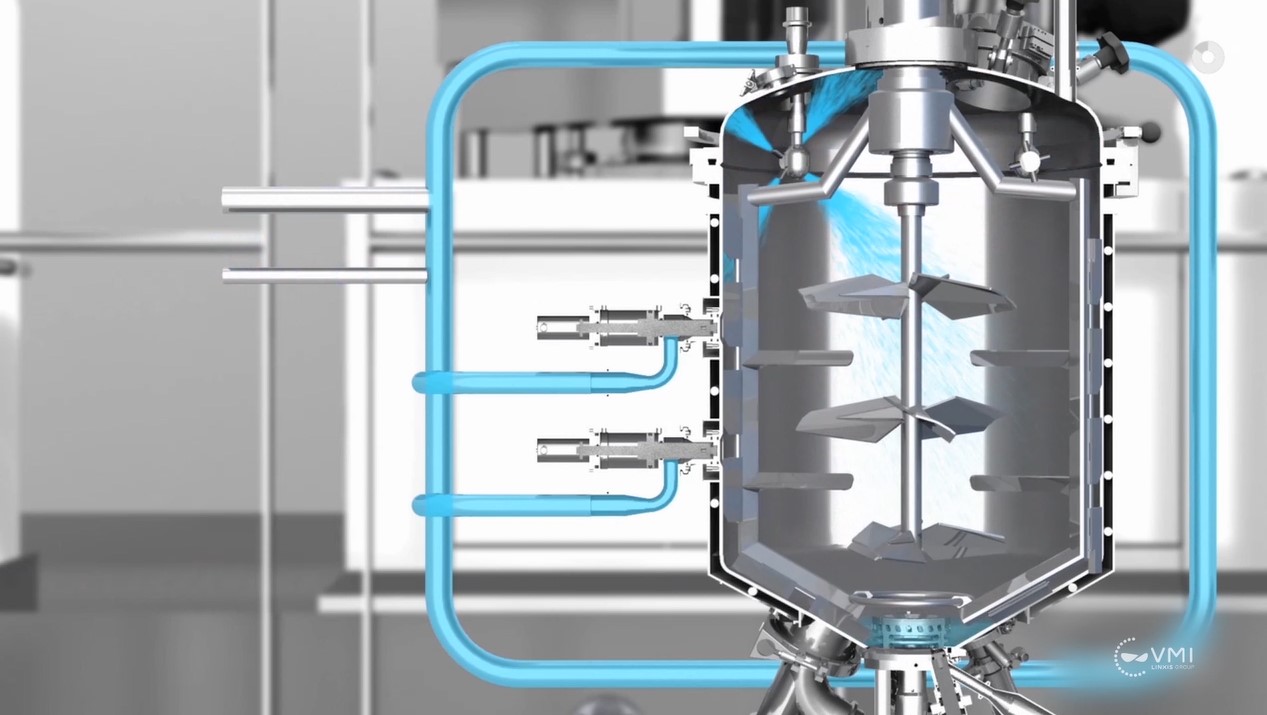
The triple agitation technology developed by VMI guarantees optimal homogenization by internal circulation loops thanks to 3 independent co-axial movements:
- Bottom agitation with an emulsifier-homogenizer ensuring a perfect dispersion of the powders and a fine emulsion (rotor-stator)
- Peripheral agitation, capable of working in both directions for homogenization and, if necessary, heat transfer (anchor blade and scrapers)
- Central axial flow agitation for homogenization and circulation (wide three-blade)

Vacuum manufacturing
Raw material introduction systems
Many chemicals, cosmetics, pharmaceutical, or food manufacturing processes involve the dispersion of solid materials in powder form within liquid or paste phases. The success of this critical homogenization phase depends on the ability of the equipment to manage two distinct hydrodynamic regimes, disperse particles and mix liquid phases simultaneously.
Vacuum introduction of powders into the heart of the emulsifier
VMI has designed and patented a rotor-stator emulsifier to allow recirculation inside the tank in two phases:
- additional vacuum facilitating the introduction and wetting of powders
- fine homogenization of powders and liquids
This design efficiently disperses particles in a mixture with a high flow rate and avoids the disadvantages of external recirculation by reducing product losses and limiting retention zones. The powders are thus introduced directly into the heart of the mixing zone, eliminating the risks of particle aggregation and powder projection through the liquid phase. The production capacity is improved, and the absence of recirculation piping facilitates cleaning.
The advantages of controlled atmosphere production
In addition to the introduction of the raw materials, mixing in a controlled atmosphere has the following advantages:
- Improved emulsion fineness and final product texture thanks to vacuum mixing
- Reproducibility of production from one batch to another, with consistent mixture quality
- Reduced production time by allowing the mixture to be degassed directly in the production tank
- Use of pressurization to assist emptying the tank at the end of production
VMI’s pressurized water loop: efficient, cost-effective thermal control
The manufacturing tanks have a double jacket for heating and cooling the product. This double envelope has two functions:
- heating the mixture to allow the emulsion of the fatty and aqueous phases
- cooling the finished product for packing or storage
VMI has developed a thermal regulation system using a pressurized water loop, which has many advantages over direct injection of steam and cold water. The control unit is composed of two separate exchangers (steam/cold water) and a recirculation pump on the double jacket of the tank.
- No thermal shocks: the temperature differences between hot and cold are limited, and the heating of the double jacket is progressive and optimal. The properties and quality of the product are preserved.
- Reduced consumption: The water is recirculated through the exchanger and returned to the cooling unit, helping to optimize energy use compared to direct injection, as the water must be cooled before being discharged. The water-loop regulation contributes to more efficient resource management. Additionally, all our control units are equipped with a float trap that recovers condensates for reuse in the steam boiler, supporting operational efficiency.
- Time-saving: no time lost between two thermal cycles. Heating starts immediately after a cooling phase or vice versa.
Cleaning and Sterilization in Place (CIP/SIP)
Hygiene regulations are becoming more and more stringent to guarantee the health and safety of consumers. The cleaning, disinfection, and even sterilization of production equipment guarantee the safety of the production process.
VMI designs efficient, integrated and automated Cleaning in Place (CIP) and Sterilization in Place (SIP) systems.
Effective cleaning is a combination of several levers:
- A set of mechanical elements (washing nozzles) within the production tank
- Appropriate water temperatures. Dedicated tanks are installed in the grey zone, where the washing water is stored, heated, and/or cooled with glycol and steam
- Automatic dosage of detergents and disinfectants, according to the acidity or alkalinity of the ingredients
- A succession of cycles to be adapted according to the product: pre-wash, soaking, recirculation washing, recirculation rinsing, wastewater rinsing, and air pushing to evacuate the water and dry the equipment
- Our CIP system also performs sterilization via steam injection
Our interface allows you to program and control the cleaning cycles and create standard cleaning routines according to the type of finished product.
The multiple benefits of a CIP system
- Quality of cleaning
- No dead zone in the tank
- No retention zone in the pipes – internal circulation loop – and control of fluid speed
- Production quality
- Avoid non-conformities
- Eliminates the risk of cross-contamination or microbial contamination
- Repeatability of processes
- Automation of the cleaning process via the interface
- Recipes: automatic recording and sequencing of washing cycles
- Efficient and controlled resource usage
- Water consumption is carefully managed, with no risk of overuse compared to manual washing
- Control and optimization of detergent usage
- Reduction of non-productive cleaning time
- Safety and security
- Safe implementation, with little or no human intervention
- Verified cleaning quality at the end of CIP: no detergent is present before launching a new production cycle

Equipment safety and qualification
VMI’s mixing technologies are compliant with the standards and regulations of the pharmaceutical and cosmetics industry.
Throughout the fit-out phase, we carry out all the essential steps to integrate the equipment in its environment and assure its long-term operation: validation of the PID, functional analysis, compliance with GMP, risk analysis and management, 21 CFR Part 11 regulations, and GAMP 5.
All phases, controls and tests are carried out to meet the requirements and complete documentation for qualification of the installation (IQ) and operational qualification (OQ).
The industrial installations are fully assembled and tested in our workshops to meet the Factory Acceptance Test (FAT). You can attend and participate in the FAT in person or remotely. The equipment is then disassembled, shipped, and reassembled by our teams on the manufacturing site. Another series of tests is performed to validate the final configuration of the equipment: Site Acceptance Test (SAT).